Treatment Resistant Depression - Phase 1/2 Trial with IV DMT
Trial Design
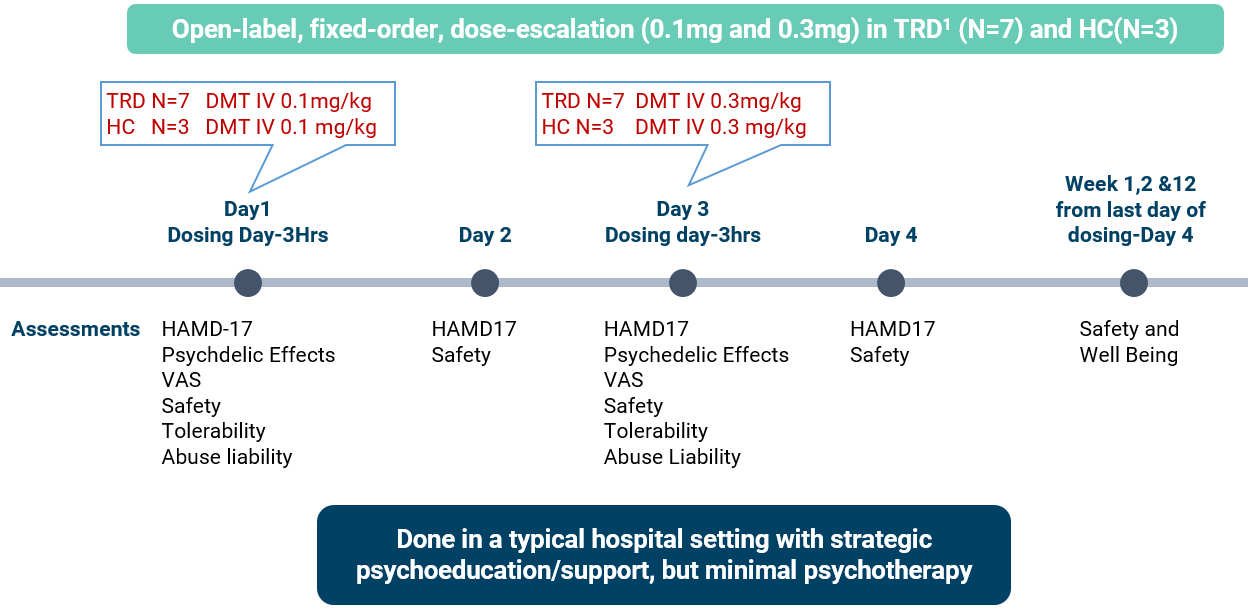
Subjects in trial included patients with TRD that have been suffering for more than 10 years.

Subjects in trial included patients with TRD that have been suffering for more than 10 years.
Safety, Tolerability and Efficacy
- Tolerabllity:
A) DMT was tolerated by both HC (n=3) and MDD participants (n=7) studied; there were no dropouts. - Safety:
A) DMT increased blood pressure, heart rate, which resolved within 20–30 min of injection. - Adverse Events:
A) Adverse events were mostly mild with one self-limited serious event. (patient with hisory of reflex syncope)
B) DMT increased anxiety which resolved within 20–30 min of injection. - Subjective Effects:
A) DMT increased psychedelic effects, and psychotomimetic effects, which resolved within 20–30 min of injection. - Abuse Liability and Drug reinforcement:
There were no dose-related differences in measures of drug reinforcement and abuse liability. - Efficacy:
A) HAMD-17 scores decreased significantly by - 4.5 (mean difference) compared to baseline in MDD participants the day after receiving 0.3 mg/kg DMT.
B) Reductions in depression were observed the day after DMT dosing, In contrast to other psychedelic treatment models.
C) Persistent effect - reduction in HAMD17 scores -1 week later (data not captured in completed open label trial, data expected in trial underway).

Treatment Resistant Depression - Second Trial with IV DMT (provisional Patent 21)
Migraine - Phase 1/2 Trial of oral Psilocybin
In an exploratory double-blind, placebo-controlled, cross-over study, adults with migraine received oral placebo and psilocybin (0.143 mg/kg) in 2 test sessions spaced 2 weeks apart. Subjects maintained headache diaries starting 2 weeks before the first session until 2 weeks after the second session. Physiological and psychological drug effects were monitored during sessions and several follow-up contacts with subjects were carried out to assure safety of study procedures. Ten subjects were included in the final analysis.
Over the 2-week period measured after single administration, the reduction in weekly migraine days from baseline was significantly greater after psilocybin (mean, - 1.65 (95% CI: - 2.53 to - 0.77) days/week) than after placebo (- 0.15 (- 1.13 to 0.83) days/week; p = 0.003, t(9) = 4.11). Changes in migraine frequency in the 2 weeks after psilocybin were not correlated with the intensity of acute psychotropic effects during drug administration.
Psilocybin was well-tolerated; there were no unexpected or serious adverse events or withdrawals due to adverse events. This exploratory study suggests there is an enduring therapeutic effect in migraine headache after a single administration of psilocybin.
The separation of acute psychotropic effects and lasting therapeutic effects is an important finding, urging further investigation into the mechanism underlying the clinical effects of select 5-HT2A receptor compounds in migraine, as well as other neuropsychiatric conditions.
Psilocybin was well-tolerated; there were no unexpected or serious adverse events or withdrawals due to adverse events. This exploratory study suggests there is an enduring therapeutic effect in migraine headache after a single administration of psilocybin.
The separation of acute psychotropic effects and lasting therapeutic effects is an important finding, urging further investigation into the mechanism underlying the clinical effects of select 5-HT2A receptor compounds in migraine, as well as other neuropsychiatric conditions.