Short Acting, Rapid Onset,
Short Acting, Rapid Onset, Sustained Efficacy Drugs for
Treatment Resistant Depression
 Short Acting, Rapid Onset,
Short Acting, Rapid Onset, Sustained Efficacy Drugs
for Treatment Resistant
Depression
Mind Pharmaceuticals is a Clinical Asset Stage Company that will target unmet needs in the CNS space with an initial focus on Treatment Resistant Depression and Headache Disorders.
MIND001, our lead candidate is an intravenous formulation of N,N-Dimethyltryptamine (DMT) targeting Treatment Resistant Depression. DMT is a naturally ocurring psychedelic found in plants and shrubs that is also speculated to be be indigenously produced in humans and is an agonist of 5H2A receptors.
Clinical Trials: An exploratory trial with 10 patients with Treatment Resistant Depression has been completed and another is underway with 60 patients with Treatment Resistant Depression.
Results: These trials indicate that MIND001 has efficacy with difficult to treat Treatment Resistant Depression, is short acting, has rapid onset and sustained efficacy, a good safety and adverse event profile, and has low addictive potential.

Efficacy with Treatment Resistant Depression
MIND001 showed Efficacy in Treatment Resistant Depression patients (which constitute 33% of patients with depression) some of them who have been suffering for upto 10 years in an exploratory trial with 10 patients which has been completed. Another trial is underway with 60 patients.This is in contrast to typical antidepressant drugs which require daily dosing.

Short Acting
The short duration of the psychedelic trip of 30 minutes is in contrast to other psychedelics in late stage trials now, like psilocybin where the psychedelic effects can last upto 8hrs.
Rapid Onset, Sustained Efficacy
Efficacy was observed the very next day in contrast to typical prescribed antidepressants in the market which take several weeks to show antidepressant effects. This is also in contrast to other psychedelic treatment models.Further it is expected the efficacy will be sustained up to several weeks to months after the day in which the patient is dosed, in the trial underway. This is in contrast to everyday dosing required of typical antidepressants.

High Tolerability, Low Abuse Potential, Good Safety Profile
There were no dropouts in the trial. Further there were no dose related differences in measures of Drug Reinforcement and Abuse Potential. The trials generally indicated good safety and adverse event profile.
Economical and Scalable Paradigm
Short Acting - The short duration of the psychedelic trip of 30 minutes is in contrast to other psychedelics in late stage trials now, like psilocybin where the psychedelic effects can last upto 8hrs.Shorter visit in an Outpatient setting - The short duration of the psychedelic trip in our completed trial implies a shorter visit to the clinic and less patient monitoring and might also allow an outpatient setting. The subjective effects of psychedelics demand patient monitoring while undergoing the trip.
No Psychotherapy - Our completed trial was conducted without any psychotherapy and with only minimal strategic psychoeducation, in contrast to typical trials with psychedelic drugs including psychotherapy.
Single Visit - Our paradigm primarily involves a single visit on dosing day. This is in contrast to typical trials with psychedelic drugs including psychotherapy and several psychotherapy associated visits.
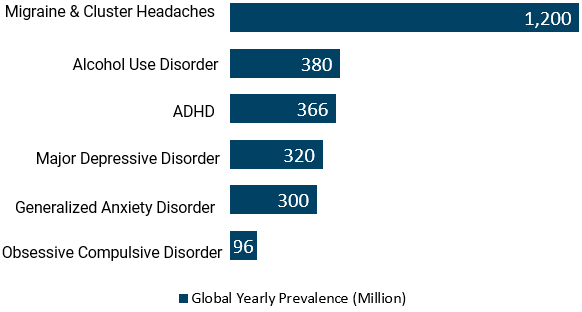
MIND will also target other large markets in the CNS space with Considerable Global Prevalence